Introduction
In chemistry laboratory, it is sometimes necessary to experimentally determine the concentration of an unknown acid or base solution. A procedure for making this kind of determination is called an acid-base titration . In this laboratory process, a solution of known concentration, called the standard solution, is carefully added to a solution of unknown concentration until the mixture becomes neutral. The neutral point of the solution is recognized by an indicator’s color change. If the unknown solution is acidic, then the standard solution will be basic. The opposite would be true if the unknown solution was basic.
We know that the mixing of equal amounts of acid and base ions will create neutral water. At the molecular level, this reaction can be illustrated with the following equation.
H + + OH - --> H 2 O
(acid) (base)
This equation states that one mole of hydrogen ions (acid) will neutralize one mole of hydroxide ions (base). Since we can exactly measure the moles of the standard solution, we can assume that the moles of the solution of unknown concentration will be the same at the neutral point. This is called the end-point of the titration. Using the equation M a V a = M b V b , we can use the experimental data from the titration to find the unknown concentration.
Objective:
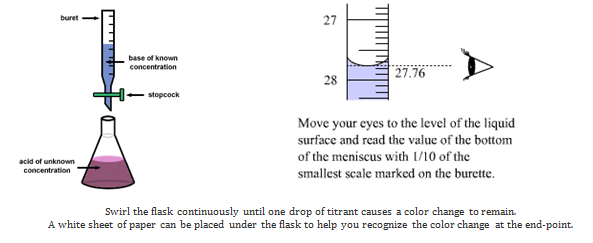
In this experiment the unknown solution will be HCl(aq) and the standard solution will be the base sodium hydroxide. You will know the concentration of the base and the volume of the acid and base used. With this information you can use the titration formula to calculate the concentration of the acid. The diagram below shows the set up.

Define the following words:
Titration- ___________________________________________________________
Endpoint- ___________________________________________________________
Neutralization- _______________________________________________________
Write the neutralization reaction for HCl reacting with NaOH.
What is the pH of the solution at the end point of the titration?
Procedure:
Calculation:
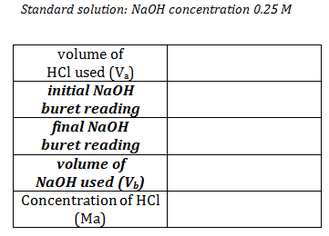
Determine the Molarity (concentration) of HCl using the data you collected and the titration formula. (The concentration of the NaOH used was 0.25M) Record your answer on your data form.